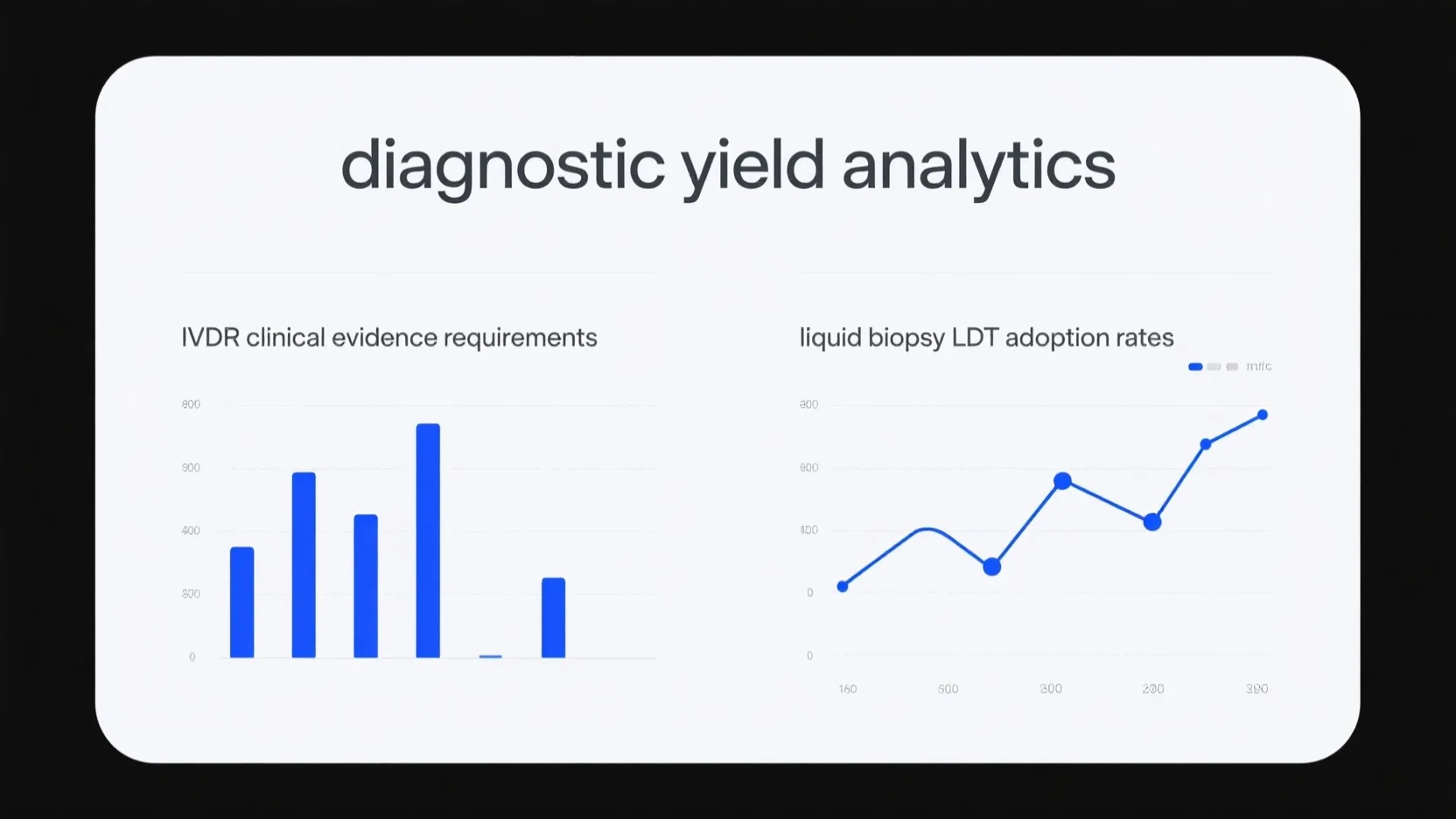
In today’s medical landscape, understanding diagnostic yield analytics, IVDR requirements, and liquid biopsy LDT adoption rates is...
CLIA-Certified Genetic Testing Solutions
In the rapidly evolving landscape of healthcare and genomics, staying ahead of the curve is crucial. Our...
In today’s rapidly evolving genomics and medical device landscape, making informed decisions is crucial. A recent SEMrush...
Looking for the best ctDNA monitoring protocols, IVD manufacturing GMP checklist, or diagnostic test CPT code mapping?...
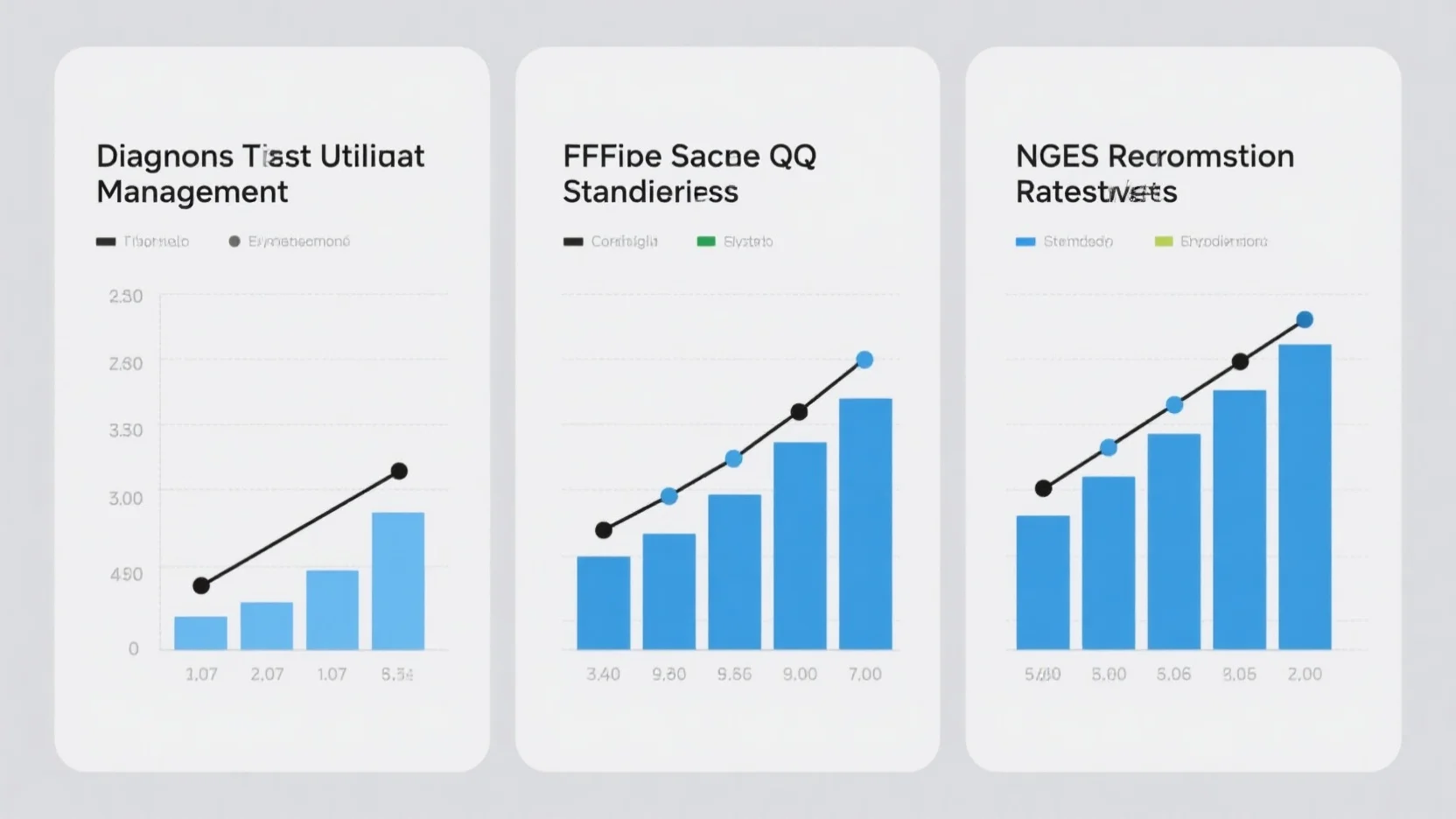
In the dynamic healthcare landscape, understanding FFPE sample QC standards, NGS reimbursement rate trends, and diagnostic test...
In the ever – advancing field of cancer diagnostics, choosing the right solid tumor sequencing panels, navigating...